Hot Sale! Osteoarthritis Knee Injection
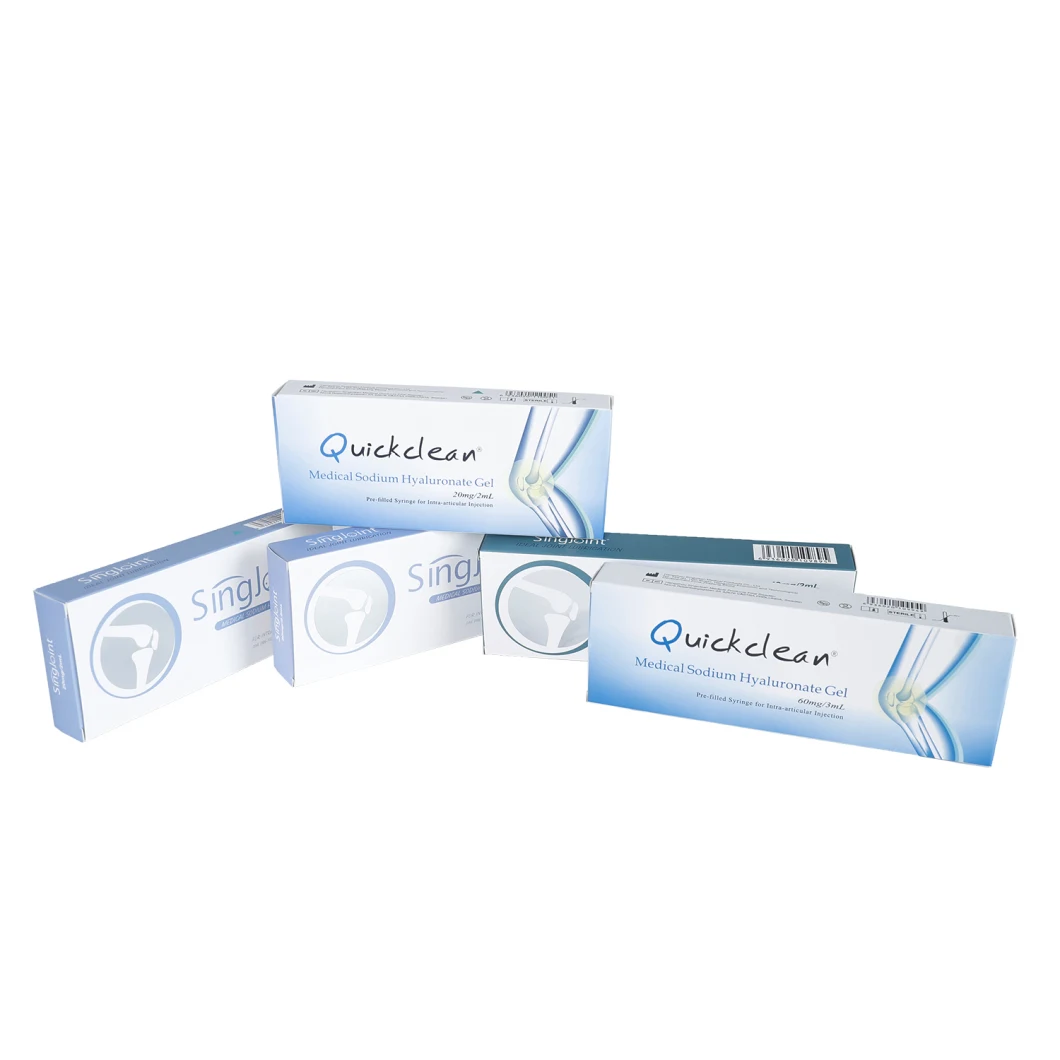
Quick Details
- Properties: Orthopedic Surgical Instruments
- Type: Implantation Equipments, Implantation Equipments
- Brand Name: Quickclean
- Model Number: QC-10-2.0
- Place of Origin: Zhejiang, China (Mainland)
- Specification: 10mg/ml
- Color: transparent
- Volume: 1ML, 2ML, 2.5ML as requested
- certification: CE,ISO
- content: Sodium Hyaluronate
- Grade: Pharm Grade
- Bacteria: None
- Application: OA Knee, pain relieve/bone joint implant
Applications:
Medical sodium hyaluronate gel is widely used as joint lubrication and for protecting the articular cartilage,
assisting the treatment of bone joint disease.
1). For Orthopedics surgery: Spread the product with syringe on the tendon nerve, around the nerve root or
inject to the joint cavity, 1ml or 2ml each time.
2) For Joint cavity: The injected quantity must be adapted to the type of surgical procedures. Once a week,
four times for one course of treatment.
| Modle No. | Volume | Concentration | Dosage |
| QC-10-1.0 | 1.0ml | 10mg/ml | 3-4 injections for one treatment course |
| QC-10-2.0 | 2.0ml | 10mg/ml | |
| QC-10-2.5 | 2.5ml | 10mg/ml | |
| QC-12-1.0 | 1.0ml | 12mg/ml | |
| QC-12-2.0 | 2.0ml | 12mg/ml | |
| QC-12-2.5 | 2.5ml | 12mg/ml | |
| QC-15-2.0 | 2.0ml | 15mg/ml | |
| QC-20-2.0 | 2.0ml | 20mg/ml | Single injection for one treatment course |
| QC-20-3.0 | 3.0ml | 20mg/ml |
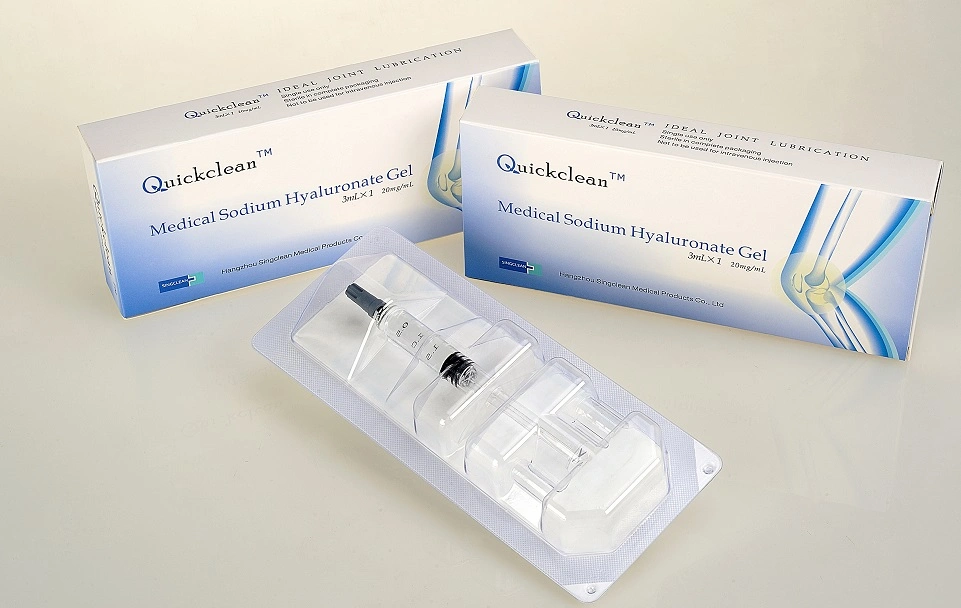
Quick Details
- Properties: Orthopedic Surgical Instruments
- Type: Implantation Equipments, Implantation Equipments
- Brand Name: Quickclean
- Model Number: QC-20-3.0
- Place of Origin: Zhejiang, China (Mainland)
- Specification: 20mg/ml
- Color: transparent
- Volume: 2ML, 3ML as requested
- certification: CE,ISO
- content: Sodium Hyaluronate
- Grade: Pharm Grade
- Bacteria: None
- Application: OA Knee, pain relieve/bone joint implant
Certification:
CE, ISO9001, ISO13485, SFDA.
Indication:
1 This product is a sterile, no pyrogen products, should be operated under strict environment .
2.disposable use; prohibite to use, once packing broken.
3.This product can't be mixed with geramine, in case being turbid.
4. intravenous injection forbidden.
5.during injection ,please inject to articular cavity ,not synovium or ligament in case more pain happen.
Disease forbidden use
Acute or chronic purulent arthritis, synovium ,tuberculosis of joint ,Hemorrhagic disease ,hemophilia ,all the diseases above are forbidden to use .
Storage:
Should place under this condition: relative humidity less than 80%, no corrosivity air , cool, dry ,ventilated , clean environment.
Why chose Quickclean hyaluronic acid joint injections ?
1.We are manufacture specilized for this product for years, so we can ensure the stable quality.
2.Our quality is approved by customers with good clinical effect.
3.Our price is competitive compared with other brands.
4.We accept OEM orders,can produce according to your requirements.
Important Safety Information
A. Strict aseptic administration technique must be followed. Free from cross infection;
B. The pre-filled syringe is intended for single use. The conents of the syringe must be used immediately after the packaging is opened. Discard any unused Quickclean®
Do not use if the blister package has been opened or damaged, or if there are cracks or breakage in the pre-filled syringe;
C. Must not be in contact with drugs containing benzalkonium chloride in order to avoid chaos;
D. Must not inject Quickclean® intravascularly;
E. Must not inject Quickclean® extra-articularly or into the synovial tissue and capsule;
F. Removejoint effusion, if present, before injection Quickclean® ;
G. Avoid concomitantly using disinfectants containing quaternary ammonium salts for skin preparation because sodium hyaluronate can precipitate in their presence.
Hangzhou Singclean Medical Products Co.Ltd
Company Introduction
Hangzhou Singclean Medical Products Co., Ltd., established in March 2003 with registered capital of 80,000,000 RMB, is a national high and new technology enterprise integrating the R&D, production and sales of medical biomaterials. With well-qualified staff, thorough quality management system and first-class facilities, the company ensures the continuous production of medical devices and drug injection products of Class III that confirm to the laws and regulations of the State Food and Drug Administration (SFDA) and EU MDD.

Interntional Sales
Welcome to try our products and visit us!